Research Plan
Interface between normal and transformed epithelial cells
Cell transformation arises from the activation of oncoproteins and/or inactivation of tumour suppressor proteins. The molecular mechanisms whereby these mutations cause cell transformation have been intensively studied by many researchers, and a variety of cell-autonomous signalling pathways have been revealed. On the other hand, the fact that cancer cells live in “a society of normal cells” has been overlooked or neglected in most studies. During the initial stage of carcinogenesis, transformation occurs in a single cell within an epithelial monolayer, however it remains unclear what happens at the interface between normal and transformed cells during this process. Do surrounding normal cells, for example, recognise the transformation that has occurred in their neighbour? What is the fate of the transformed cell when surrounded by normal cells? Using newly established cell culture systems, we have found that the interaction with surrounding normal epithelial cells affect signalling pathways and behaviour of transformed cells. One of our recent studies is introduced below.
Characterization of the interface between normal and Ras-transformed epithelial cells
(Hogan et al., 2009, Nature Cell Biology)
Ras is one of the best-characterized oncogenes, and the mutations in the Ras gene are found in more than 30% of malignant human tumours. To characterize the interface between normal and Ras-transformed epithelial cells, we established MDCK epithelial cells expressing GFP-tagged constitutively active oncogenic Ras (RasV12) in a tetracycline-inducible manner (MDCK-pTR GFP-RasV12). To examine the fate of a single RasV12 cell in a monolayer of non-transformed MDCK cells, MDCK-pTR GFP-RasV12 cells were labelled with fluorescent dye (CMPTX) and mixed with normal MDCK cells at a ratio of 1:100. The mixture of cells was then cultured on collagen matrix in the absence of tetracycline until a monolayer was formed. Subsequently, GFP-RasV12 expression was induced with tetracycline and the fate of RasV12 cells surrounded by normal cells was observed. Within 13-25 h after tetracycline addition, the RasV12 cells were often extruded from the apical surface of the monolayer (Fig. 1a).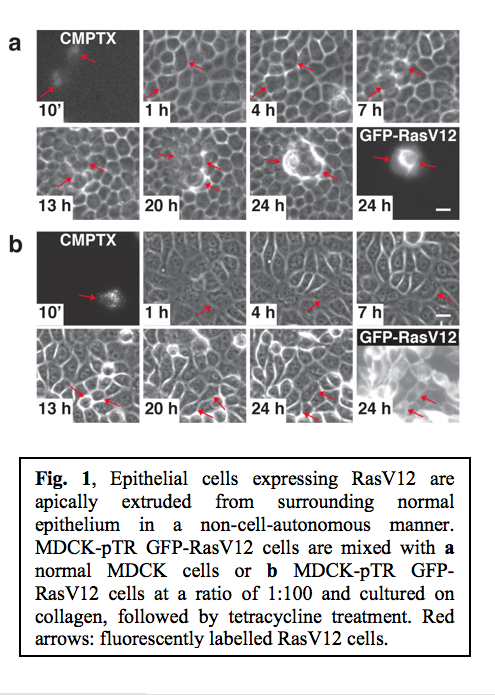 After extrusion, RasV12 cells continued to proliferate and formed multi-cellular aggregates (Fig. 1a). Importantly, fluorescently labelled RasV12 cells were not extruded when mixed with non-stained RasV12 cells (Fig. 1b), suggesting that the extrusion of RasV12 cells depends on the interaction with non-transformed cells. These data indicate that the extrusion of RasV12 cells occurs in a non-cell-autonomous manner, only when they are surrounded by normal cells.
After extrusion, RasV12 cells continued to proliferate and formed multi-cellular aggregates (Fig. 1a). Importantly, fluorescently labelled RasV12 cells were not extruded when mixed with non-stained RasV12 cells (Fig. 1b), suggesting that the extrusion of RasV12 cells depends on the interaction with non-transformed cells. These data indicate that the extrusion of RasV12 cells occurs in a non-cell-autonomous manner, only when they are surrounded by normal cells.
Although the majority of RasV12 cells were apically extruded when surrounded by normal cells, some of RasV12 cells were not extruded. We observed that non-extruded RasV12 cells produced large protrusions that dynamically extended and retracted, often over distances of several cell diameters (Fig. 2). 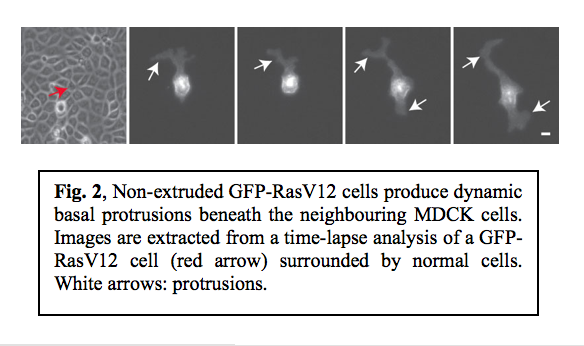 Confocal microscopy analyses revealed that these protrusions extended beneath the neighbouring non-transformed cells. When expression of RasV12 was induced in a group of cells within a monolayer of normal cells, major protrusions were frequently formed at the interface between RasV12 and non-transformed cells, but were rarely observed between RasV12 cells. This indicates that protrusion formation also depends on the interaction between normal and RasV12 cells.
Confocal microscopy analyses revealed that these protrusions extended beneath the neighbouring non-transformed cells. When expression of RasV12 was induced in a group of cells within a monolayer of normal cells, major protrusions were frequently formed at the interface between RasV12 and non-transformed cells, but were rarely observed between RasV12 cells. This indicates that protrusion formation also depends on the interaction between normal and RasV12 cells.
In summary, when RasV12 is expressed in single cells within an epithelial monolayer, two independent phenomena can occur in a non-cell-autonomous manner (Fig. 3). 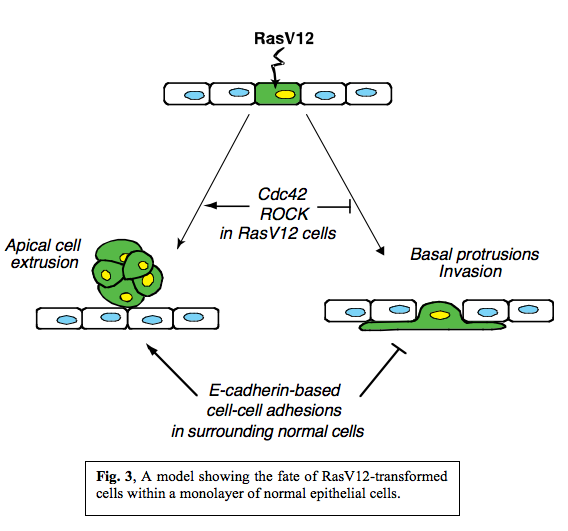 RasV12-expressing cells are either apically extruded from the monolayer or form basal protrusions leading to basal invasion into a matrix. We also showed that various signalling molecules including Cdc42 and Myosin-II were activated in RasV12 cells when they were surrounded by normal cells and that the fate of RasV12 cells is influenced by activity of Cdc42 and ROCK in RasV12 cells and by E-cadherin-based cell-cell adhesions in the surrounding normal cells. Thus, the total balance of multiple signalling pathways in normal and RasV12 cells affects the fate of RasV12 cells in the epithelial monolayer, and RasV12 cells leave epithelial sheets either apically or basally in a cell-context-dependent manner.
RasV12-expressing cells are either apically extruded from the monolayer or form basal protrusions leading to basal invasion into a matrix. We also showed that various signalling molecules including Cdc42 and Myosin-II were activated in RasV12 cells when they were surrounded by normal cells and that the fate of RasV12 cells is influenced by activity of Cdc42 and ROCK in RasV12 cells and by E-cadherin-based cell-cell adhesions in the surrounding normal cells. Thus, the total balance of multiple signalling pathways in normal and RasV12 cells affects the fate of RasV12 cells in the epithelial monolayer, and RasV12 cells leave epithelial sheets either apically or basally in a cell-context-dependent manner.
The most crucial findings in this study are that RasV12-transformed cells are able to recognize a difference(s) in the surrounding normal cells and that the presence of surrounding normal cells influences various signalling pathways and behaviours of RasV12 cells.
Future plan
1) Characterization of the interface between normal and other types of transformed cells.
Our preliminary results suggest that the presence of surrounding normal cells influences signalling pathways and fate of other types of transformed cells, leading to apical extrusion or apoptosis of transformed cells. To understand molecular mechanisms, detailed characterization and analysis of these phenomena will be required.
2) Identification and characterization of molecules that are involved in cell recognition between normal and transformed cells and in various phenomena occurring at the interface.
The most fascinating question is how cells “sense” the differences with their neighbours and how various signalling pathways are regulated accordingly. To investigate these molecular mechanisms, we are currently using two screening methods: proteomic approach and cDNA microarray.。
3) Establishment of in vivo systems: Drosophila and Zebrafish.
Another vital question is whether the phenomena that we have observed in cell culture systems also occur in vivo. We are going to utilize Drosophila melanogaster and zebrafish systems to this end.